Abstract
Introduction: Myelodysplastic syndromes (MDS) are a spectrum of clonal bone marrow failure disorders demonstrating cytopenias, dysplasia, and risk of progression. Although ~50% of MDS patients have normal karyotype (NK), a subset of MDS exhibits genetic alterations that result in an abnormal karyotype. However, the relationship between the molecular landscape and histologic features in MDS have not been well explored. Using next generation sequencing (NGS) and cytogenetics, we sought to correlate genetic abnormalities in MDS with morphologic dysplasia.
Methods: After IRB approval, we retrospectively identified NGS studies performed with a 164 gene panel in patients with MDS at Stanford (2018-2021). Molecular and cytogenetic data were collected for 225 MDS patients. Peripheral blood and bone marrow (BM) slides were collected from 61 patients for histological review. As controls, 32 staging BMs were obtained from untreated lymphoma patients.
A hematopathologist performed a blinded intense morphologic review. A subset of cases was reviewed by 2 other hematopathologists and the intraclass correlation coefficient (ICC) was calculated to evaluate concordance. The degree of dysplastic features and overall dysplasia was scored.
The standardized mean difference (SMD) was calculated to quantify the degree of dysplasia between comparison groups. Statistical analysis was performed using R (3.6.3); p value < 0.05 was considered statistically significant.
Results: MDS cases had higher degrees of dysplasia than control cases in megakaryocytic (median 37.5% vs 0%), granulocytic (median 20% vs 0%), and erythroid (median 10% vs 0%) lineages. To assess specificity, using a cutoff of 10%, 1/31 control cases (3.1%) showed megakaryocytic dysplasia, 2/32 (6.3%) had erythroid dysplasia, while none showed granulocytic dysplasia. Dysplasia in > 2 lineages in >10% of cells was specific for MDS, detected in 49/61 (80.3%) of MDS cases vs 0/32 control cases (Table 1).
Within the MDS group, lack of multilineage dysplasia was more common in NK (8/25; 32%) vs non-NK (3/36; 8.3%), suggesting MDS-NK is morphologically subtle. Using a cutoff of 10%, 13/25 (52%) NK lacked granulocytic dysplasia vs 10/36 (27.8%) in non-NK, 8/25 (32%) of NK lacked erythroid dysplasia vs 10/36 (27.8%) in non-NK, and 5/25 (20%) NK lacked megakaryocytic dysplasia vs 3/35 (8.3%) in non-NK.
The most strongly associated dysplastic features for MDS included erythroid megaloblastic change (SMD=1.25), erythroid nuclear features (SMD=1.041), atypical megakaryocyte nuclei (SMD=1.189), separated megakaryocyte nuclei (SMD=1.131), and hypogranulated granulocytes (SMD=1.096).
Based on 10 MDS and 5 control cases, rough concordance on overall dysplasia was demonstrated among 3 hematopathologists. Granulocytic dysplasia showed the highest reproducibility (ICC for granulocytic = 0.948, erythroid = 0.547, megakaryocytic = 0.531).
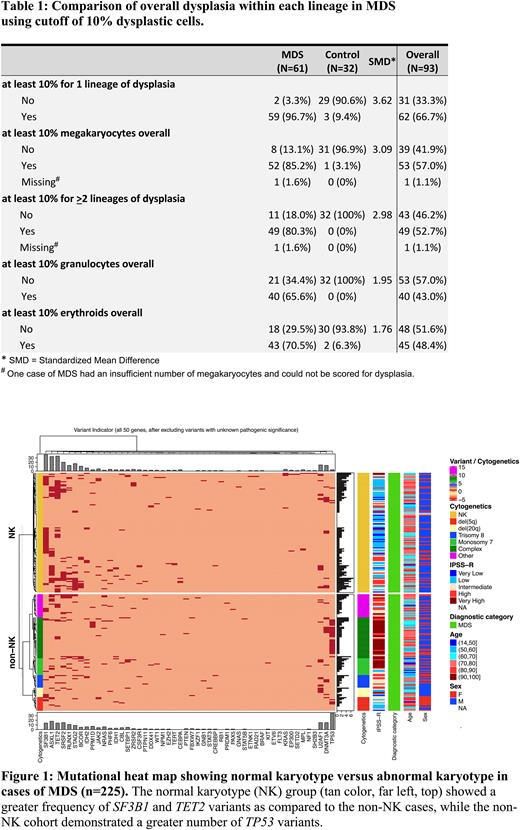
The percent of cells with dysplasia in the MDS cohort did not correlate with number of mutations. The overall number of mutations between MDS with NK and non-NK were relatively similar (Figure 1). The non-NK group exhibited a greater frequency of pathogenic TP53 variants than NK (31.5% vs 2.6%, p < 0.0001). Conversely, SF3B1 and TET2variants occurred more frequently in the NK cohort than non-NK (SF3B1: 32.5% vs 12.6%; TET2: 29.8% vs 12.6%, both p values < 0.003). In MDS cases with a TET2 variant, NK was associated with a significantly higher total number of mutations than non-NK cases (median [IQR]: 5[5-7] vs 4[3-6], p = 0.031).
Using Random Forest models, exploratory multivariate analysis identified the presence of mutations in TP53, SF3B1, TET2, and overall megakaryocytic, granulocytic, and erythroid dysplasia as the prominent parameters that distinguished NK from non-NK MDS cases.
There was no significant difference in age at diagnosis between NK and non-NK MDS in all NGS cases (median age 74 vs 70, p = 0.10) and morphologically scored cases (median age 70 in both groups).
Conclusion: Our study demonstrates that granulocytic dysplasia was the most specific morphologic finding for MDS with high reproducibility among 3 hematopathologists. Detection of dysplasia in > 2 lineages in >10% cells was also specific for MDS, while MDS-NK appear morphologically subtle. Mutations in TET2 were more frequent in MDS-NK. Our findings highlight the importance of NGS and histopathology in helping establish a diagnosis of MDS-NK.
Disclosures
Fernandez-Pol:Cartography Biosciences: Consultancy; Leica Biosystems: Ended employment in the past 24 months, Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal